Hydrogen Fuel Cells: An Environmentally Friendly Alternative
Calculations Run at NERSC Point to Polymer Membrane Advantages
March 29, 2022
Contact: cscomms@lbl.gov
In the quest to find alternatives to fossil fuels for powering cars, trucks, sea-going vessels, and other methods of transport, much attention is being paid to next-generation batteries and the infrastructure needed to support electric vehicles. But an equally viable approach – hydrogen fuel cells – is finding favor, and funding, as well.
Hydrogen fuel cells are an environmentally friendly technology that converts hydrogen into electricity through an electrochemical process that emits only water and heat as byproducts. With this in mind, in 2021 the U.S. Department of Energy (DOE) launched the Energy Earthshots Initiative, with the first Energy Earthshot aimed at reducing the cost of clean hydrogen by 80% to $1 per 1 kilogram in 1 decade (the so-called “111” program).
"Fuel cells, which are seen by many as providing a route for electrification of medium and heavy duty transportation such as trucks, buses, and ships, offer key advantages over batteries – including better scaling of power with weight and energy, which enables them to overcome the current range and weight challenges of batteries," said Adam Weber, senior scientist and hydrogen and fuel cell technologies lab program manager at Lawrence Berkeley National Laboratory (Berkeley Lab).
Toward this end, several current research efforts are focused on improving the design of these clean energy devices and the materials used in their construction. In a paper published Jan. 31 in Nature Energy, an international team of researchers demonstrated how computer simulations can enhance the efficiency and durability of polymer electrolyte membrane hydrogen fuel cells by identifying specific composite polymers that address a key challenge to their use in cars, trucks, and other heavy-duty vehicles.

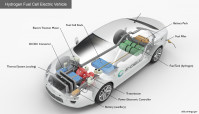
Polymer electrolyte membrane (PEM) fuel cells used in automobiles—also called proton exchange membrane fuel cells—use hydrogen fuel and oxygen from the air to produce electricity. (Credit: DOE Office of Energy Efficiency & Renewable Energy)
This study included computations run on the Cori supercomputer at the National Research Energy Scientific Computing Center (NERSC) at Berkeley Lab and supercomputing resources at Oak Ridge National Laboratory (ORNL). The calculations run at NERSC were conducted through an allocation in support of research carried out by the collaborative Center for Predictive Simulation of Functional Materials at ORNL.
“In materials research, computations are often used when you need to explain some experimental data that would otherwise be difficult to understand, or to predict certain properties that help experimentalists narrow the search space for a certain material or property,” said Ivana Gonzales (née Ivana Matanovic), research associate professor at the University of New Mexico, a scientist at Los Alamos National Laboratory, and co-author on the Nature Energy paper. “We use the calculations to guide the experimental team in the design of specific materials and devices.”
No Greenhouse Gases
Fuel cells offer some key advantages over batteries, including higher energy density by weight, which enables them to overcome the current range and weight challenges of batteries. Hydrogen fuel cells are even more attractive when it comes to their environmental impact, Gonzales noted.
“If the hydrogen being used is produced from electricity from green sources, then you have green hydrogen and you have fuel cells that produce no greenhouse gas emissions,” she explained. “So these fuel cells offer a transition to an environmentally friendly, hydrogen-based economy.”
For the Nature Energy paper, the research collaboration – which included scientists and engineers from the U.S., Republic of Korea, and Germany – focused on the polymers used in high-temperature polymer electrolyte membrane fuel cells (HT-PEMFCs). Currently available hydrogen fuel cells typically use phosphoric acid-doped polybenzimidazole in the membranes, but these cells tend to experience a loss of phosphoric acid when operating below 140 degrees centigrade or during frequent startups/shutdowns, making them unfavorable for automotive applications. This challenge prompted the study’s computational researchers to look for other options.
For Gonzales, this meant applying density functional theory (DFT) calculations to three different components of the experimental work:
- To explain the structure of the composite polymers. “We can calculate spectroscopic data and marked spectra more specifically, which helps explain what is actually happening in these composite ionomers, which in turn leads to designing better performing materials and devices,” Gonzales said.
- To understand the chemical processes that occur in mixed polymers. Gonzales calculated the energetics of proton transfer to reveal the redistribution of protons between different components of the ionomers. “Conductivity depends on the protons; if you don’t have conductivity in the membrane between the electrodes, then the fuel cell won’t work,” she explained. “So maintaining good conductivity during long operation time of the device is extremely important. The DFT calculations helped explain the kinetics of the process and provide data for the team to understand what is happening in these systems.”
- To calculate the durability of the fuel cell devices. “Ionomers are dotted with acid molecules, and these acids are the source of protons, which are very important for conductivity of the system,” Gonzalez said. “But this ability can be decreased if these acids form on anhydrides at high temperatures. Based on our calculations, we postulated that protonated ionomers will have less tendency to form the acid anhydrides, yielding higher stability and thus extending operation times.”
These calculations led the team to determine that when a proton is added to an atom, molecule, or ion – thus forming a conjugate acid – the resulting protonated phosphonic acid can increase proton conductivity by more than an order of magnitude compared with a non-protonated phosphonic acid.
“We show experimental and theoretical evidence of the protonation of phosphonic acids that is distinct from the hydrogen bonding of phosphonic acids,” the researchers wrote. Based on this concept, they designed protonated phosphonic acid electrodes that enable “remarkable power density and are well suited for HDV (heavy-duty vehicle) fuel cells.”
“Our emphasis is on getting the results, designing new materials, and reporting on a fuel cell that is much more advanced,” Gonzales said. “In this case, the team reported higher rated power densities than current state-of-the-art fuel cells, which is very new.”
Commercial Interest
This technology is being pursued for commercial application through DOE’s ARPA-E program and the Hydrogen and Fuel Cell Technologies Office within the Energy Efficiency and Renewable Energy Office. But investment in fuel cell R&D is not coming just from government and academic sources. According to the U.S. Environmental Protection Agency, several auto manufacturers are selling or leasing fuel cell vehicles in select markets, primarily in California where some hydrogen fueling stations already exist. Hydrogen infrastructure is also popping up in other locations around the country; stations are being planned or built in the Northeast and Hawaii, and fuel cell transit buses are already cruising the streets in cities like Boston and Flint, Michigan.
“A lot of companies are working on fuel cells for cars and trucks, and we are working with many of them,” Gonzales said. “This particular project was funded in part by ARPA-E, and part of that ARPA-E team includes Toyota and Advent Technologies. There is much interest in commercializing these devices and the components for these devices.”
While there continues to be much discussion about which technologies will be best to create a clean energy economy, “when it comes to whether to use batteries or fuel cells [for vehicles], the consensus is we will probably be using both,” Gonzales noted. “Hydrogen fuel cells have pretty good efficiency – larger than an internal combustion engine – but we are working to reduce the cost of manufacturing.”
This research illustrates how important supercomputers such as those at NERSC and ORNL have become in applied science, she added. “This is the reality now: scientists are routinely using computers to help design materials for a variety of applications and devices.”
About Computing Sciences at Berkeley Lab
High performance computing plays a critical role in scientific discovery. Researchers increasingly rely on advances in computer science, mathematics, computational science, data science, and large-scale computing and networking to increase our understanding of ourselves, our planet, and our universe. Berkeley Lab’s Computing Sciences Area researches, develops, and deploys new foundations, tools, and technologies to meet these needs and to advance research across a broad range of scientific disciplines.







 Instagram
Instagram YouTube
YouTube